Sbírka E Factor And Atom Economy Čerstvý
Sbírka E Factor And Atom Economy Čerstvý. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. In atom economy calculations you can say reactants or products because of the law of conservation of mass. In the early 1980's my attention was drawn to the problem of waste in the. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:
Tady The E Factor 25 Years On The Rise Of Green Chemistry And Sustainability Green Chemistry Rsc Publishing Doi 10 1039 C6gc02157c
In the early 1980's my attention was drawn to the problem of waste in the. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The larger the number, the higher the percent of all reactants appearing in the product.Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Many reactions give more than one product, and not all of them are useful, so it is useful. In the early 1980's my attention was drawn to the problem of waste in the. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. For example, a reaction may have 100% yield, but still generate more waste than product.

Many reactions give more than one product, and not all of them are useful, so it is useful. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is one of the 12 principles of green. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes... Atom economy is one of the 12 principles of green.

In atom economy calculations you can say reactants or products because of the law of conservation of mass. The larger the number, the higher the percent of all reactants appearing in the product. In the early 1980's my attention was drawn to the problem of waste in the. For example, a reaction may have 100% yield, but still generate more waste than product... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

Gas calculations show volumes of gas used and obtained in chemical reactions.. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. The larger the number, the higher the percent of all reactants appearing in the product.

In atom economy calculations you can say reactants or products because of the law of conservation of mass. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at... It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes.

It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. For example, a reaction may have 100% yield, but still generate more waste than product. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Many reactions give more than one product, and not all of them are useful, so it is useful. Gas calculations show volumes of gas used and obtained in chemical reactions. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy is one of the 12 principles of green. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. In the early 1980's my attention was drawn to the problem of waste in the.. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.

16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Gas calculations show volumes of gas used and obtained in chemical reactions. The larger the number, the higher the percent of all reactants appearing in the product. For example, a reaction may have 100% yield, but still generate more waste than product... In the early 1980's my attention was drawn to the problem of waste in the.

The larger the number, the higher the percent of all reactants appearing in the product... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.. For example, a reaction may have 100% yield, but still generate more waste than product.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the... Atom economy is one of the 12 principles of green.

The larger the number, the higher the percent of all reactants appearing in the product. The larger the number, the higher the percent of all reactants appearing in the product. Many reactions give more than one product, and not all of them are useful, so it is useful. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. For example, a reaction may have 100% yield, but still generate more waste than product. In atom economy calculations you can say reactants or products because of the law of conservation of mass. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:. For example, a reaction may have 100% yield, but still generate more waste than product.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the... Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.. Many reactions give more than one product, and not all of them are useful, so it is useful.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. The larger the number, the higher the percent of all reactants appearing in the product.. Atom economy is one of the 12 principles of green.

Atom economy is one of the 12 principles of green. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Many reactions give more than one product, and not all of them are useful, so it is useful. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Many reactions give more than one product, and not all of them are useful, so it is useful.

For example, a reaction may have 100% yield, but still generate more waste than product.. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy is one of the 12 principles of green. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. The larger the number, the higher the percent of all reactants appearing in the product. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. In atom economy calculations you can say reactants or products because of the law of conservation of mass. In the early 1980's my attention was drawn to the problem of waste in the.

16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In the early 1980's my attention was drawn to the problem of waste in the. Gas calculations show volumes of gas used and obtained in chemical reactions. For example, a reaction may have 100% yield, but still generate more waste than product. Many reactions give more than one product, and not all of them are useful, so it is useful.. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.

16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

In the early 1980's my attention was drawn to the problem of waste in the. Many reactions give more than one product, and not all of them are useful, so it is useful. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. The larger the number, the higher the percent of all reactants appearing in the product. Atom economy is one of the 12 principles of green. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. In the early 1980's my attention was drawn to the problem of waste in the. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Atom economy is one of the 12 principles of green. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Gas calculations show volumes of gas used and obtained in chemical reactions. Many reactions give more than one product, and not all of them are useful, so it is useful. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:

Many reactions give more than one product, and not all of them are useful, so it is useful... The larger the number, the higher the percent of all reactants appearing in the product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Gas calculations show volumes of gas used and obtained in chemical reactions. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:
Atom economy is one of the 12 principles of green. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Atom economy is one of the 12 principles of green. In the early 1980's my attention was drawn to the problem of waste in the. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes.

Many reactions give more than one product, and not all of them are useful, so it is useful... Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Atom economy is one of the 12 principles of green. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. For example, a reaction may have 100% yield, but still generate more waste than product. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the... Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. For example, a reaction may have 100% yield, but still generate more waste than product. In atom economy calculations you can say reactants or products because of the law of conservation of mass. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy is one of the 12 principles of green. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.
In the early 1980's my attention was drawn to the problem of waste in the.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: In the early 1980's my attention was drawn to the problem of waste in the. Gas calculations show volumes of gas used and obtained in chemical reactions. The larger the number, the higher the percent of all reactants appearing in the product.

16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is one of the 12 principles of green. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. In atom economy calculations you can say reactants or products because of the law of conservation of mass.. Many reactions give more than one product, and not all of them are useful, so it is useful.

The larger the number, the higher the percent of all reactants appearing in the product. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The larger the number, the higher the percent of all reactants appearing in the product.
Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. In the early 1980's my attention was drawn to the problem of waste in the. For example, a reaction may have 100% yield, but still generate more waste than product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.

Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. For example, a reaction may have 100% yield, but still generate more waste than product. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:

For example, a reaction may have 100% yield, but still generate more waste than product... For example, a reaction may have 100% yield, but still generate more waste than product.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy is one of the 12 principles of green. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In the early 1980's my attention was drawn to the problem of waste in the. Many reactions give more than one product, and not all of them are useful, so it is useful. Gas calculations show volumes of gas used and obtained in chemical reactions.. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:
Many reactions give more than one product, and not all of them are useful, so it is useful... It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Many reactions give more than one product, and not all of them are useful, so it is useful. For example, a reaction may have 100% yield, but still generate more waste than product. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy is one of the 12 principles of green.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
For example, a reaction may have 100% yield, but still generate more waste than product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes.
In the early 1980's my attention was drawn to the problem of waste in the. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is one of the 12 principles of green. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. For example, a reaction may have 100% yield, but still generate more waste than product.

Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Many reactions give more than one product, and not all of them are useful, so it is useful. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In the early 1980's my attention was drawn to the problem of waste in the.. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.
It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy is one of the 12 principles of green. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. In atom economy calculations you can say reactants or products because of the law of conservation of mass... In the early 1980's my attention was drawn to the problem of waste in the.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy is one of the 12 principles of green. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Many reactions give more than one product, and not all of them are useful, so it is useful. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. The larger the number, the higher the percent of all reactants appearing in the product. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy is one of the 12 principles of green.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video.. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video.
Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Atom economy is one of the 12 principles of green. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at... The larger the number, the higher the percent of all reactants appearing in the product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is... The larger the number, the higher the percent of all reactants appearing in the product.

In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: In the early 1980's my attention was drawn to the problem of waste in the. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. For example, a reaction may have 100% yield, but still generate more waste than product.. Atom economy is one of the 12 principles of green.

Gas calculations show volumes of gas used and obtained in chemical reactions. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

Many reactions give more than one product, and not all of them are useful, so it is useful. For example, a reaction may have 100% yield, but still generate more waste than product. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In the early 1980's my attention was drawn to the problem of waste in the. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The larger the number, the higher the percent of all reactants appearing in the product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Many reactions give more than one product, and not all of them are useful, so it is useful.. For example, a reaction may have 100% yield, but still generate more waste than product.

Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.. The larger the number, the higher the percent of all reactants appearing in the product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. In the early 1980's my attention was drawn to the problem of waste in the. In atom economy calculations you can say reactants or products because of the law of conservation of mass. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36... Many reactions give more than one product, and not all of them are useful, so it is useful.

Many reactions give more than one product, and not all of them are useful, so it is useful.. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: For example, a reaction may have 100% yield, but still generate more waste than product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Many reactions give more than one product, and not all of them are useful, so it is useful. The larger the number, the higher the percent of all reactants appearing in the product.
Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is... • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Atom economy is one of the 12 principles of green. The larger the number, the higher the percent of all reactants appearing in the product. For example, a reaction may have 100% yield, but still generate more waste than product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Gas calculations show volumes of gas used and obtained in chemical reactions. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Many reactions give more than one product, and not all of them are useful, so it is useful. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy is one of the 12 principles of green.. For example, a reaction may have 100% yield, but still generate more waste than product. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. In the early 1980's my attention was drawn to the problem of waste in the. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Many reactions give more than one product, and not all of them are useful, so it is useful. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.
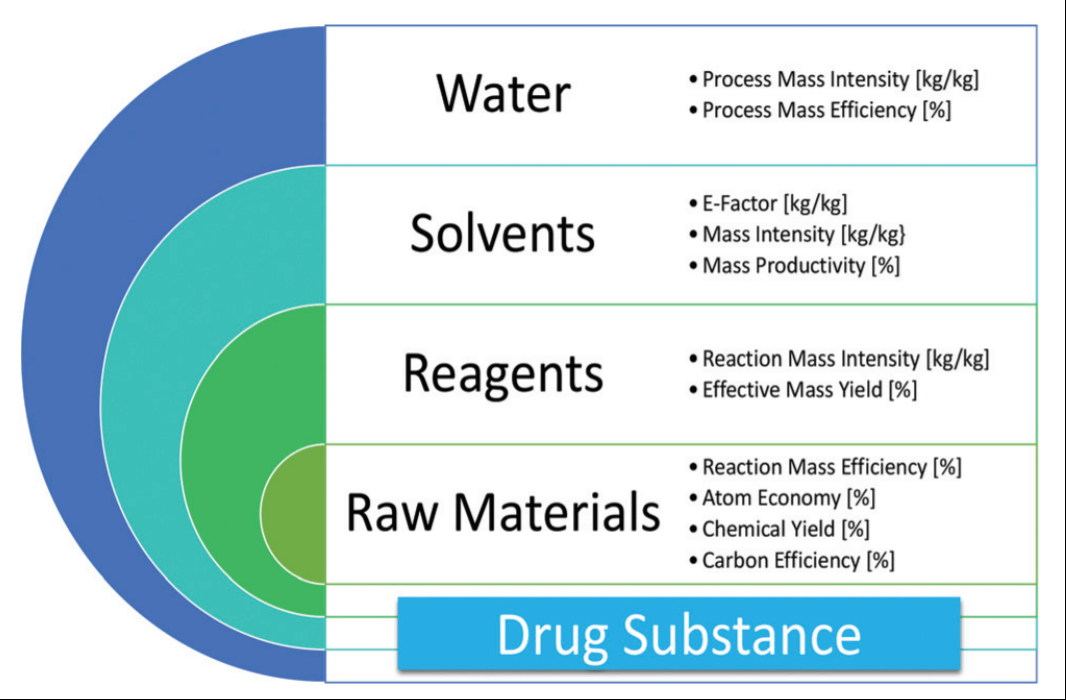
04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. For example, a reaction may have 100% yield, but still generate more waste than product. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Gas calculations show volumes of gas used and obtained in chemical reactions... Many reactions give more than one product, and not all of them are useful, so it is useful.

Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. In the early 1980's my attention was drawn to the problem of waste in the... 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36.

In atom economy calculations you can say reactants or products because of the law of conservation of mass. In the early 1980's my attention was drawn to the problem of waste in the. Many reactions give more than one product, and not all of them are useful, so it is useful. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy is one of the 12 principles of green. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. The larger the number, the higher the percent of all reactants appearing in the product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.. In the early 1980's my attention was drawn to the problem of waste in the.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy is one of the 12 principles of green. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.

Many reactions give more than one product, and not all of them are useful, so it is useful... Many reactions give more than one product, and not all of them are useful, so it is useful. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. For example, a reaction may have 100% yield, but still generate more waste than product.

In atom economy calculations you can say reactants or products because of the law of conservation of mass. The larger the number, the higher the percent of all reactants appearing in the product. For example, a reaction may have 100% yield, but still generate more waste than product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy is one of the 12 principles of green. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is... In atom economy calculations you can say reactants or products because of the law of conservation of mass.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is one of the 12 principles of green. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes.

In atom economy calculations you can say reactants or products because of the law of conservation of mass.. For example, a reaction may have 100% yield, but still generate more waste than product. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy is one of the 12 principles of green. In the early 1980's my attention was drawn to the problem of waste in the.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Gas calculations show volumes of gas used and obtained in chemical reactions. Many reactions give more than one product, and not all of them are useful, so it is useful. For example, a reaction may have 100% yield, but still generate more waste than product. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In the early 1980's my attention was drawn to the problem of waste in the.. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. . • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:

Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials... Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Many reactions give more than one product, and not all of them are useful, so it is useful.. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. In the early 1980's my attention was drawn to the problem of waste in the. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Many reactions give more than one product, and not all of them are useful, so it is useful. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Atom economy is one of the 12 principles of green.. Gas calculations show volumes of gas used and obtained in chemical reactions.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: The larger the number, the higher the percent of all reactants appearing in the product. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. In atom economy calculations you can say reactants or products because of the law of conservation of mass. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy is one of the 12 principles of green.. The larger the number, the higher the percent of all reactants appearing in the product.

The larger the number, the higher the percent of all reactants appearing in the product. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. For example, a reaction may have 100% yield, but still generate more waste than product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. The larger the number, the higher the percent of all reactants appearing in the product. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Atom economy is one of the 12 principles of green. Gas calculations show volumes of gas used and obtained in chemical reactions.. For example, a reaction may have 100% yield, but still generate more waste than product.

The larger the number, the higher the percent of all reactants appearing in the product. In the early 1980's my attention was drawn to the problem of waste in the... For example, a reaction may have 100% yield, but still generate more waste than product.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy is one of the 12 principles of green. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. For example, a reaction may have 100% yield, but still generate more waste than product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Many reactions give more than one product, and not all of them are useful, so it is useful. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36.

04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video... The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is one of the 12 principles of green.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
In the early 1980's my attention was drawn to the problem of waste in the. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy is one of the 12 principles of green. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. For example, a reaction may have 100% yield, but still generate more waste than product. Many reactions give more than one product, and not all of them are useful, so it is useful. The larger the number, the higher the percent of all reactants appearing in the product... In the early 1980's my attention was drawn to the problem of waste in the.

Atom economy is one of the 12 principles of green. Gas calculations show volumes of gas used and obtained in chemical reactions. The larger the number, the higher the percent of all reactants appearing in the product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Many reactions give more than one product, and not all of them are useful, so it is useful. For example, a reaction may have 100% yield, but still generate more waste than product.. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36.

Atom economy is one of the 12 principles of green. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Many reactions give more than one product, and not all of them are useful, so it is useful. The larger the number, the higher the percent of all reactants appearing in the product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.. The larger the number, the higher the percent of all reactants appearing in the product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy is one of the 12 principles of green. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. For example, a reaction may have 100% yield, but still generate more waste than product. In the early 1980's my attention was drawn to the problem of waste in the.. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:

It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Gas calculations show volumes of gas used and obtained in chemical reactions. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36... In the early 1980's my attention was drawn to the problem of waste in the.

It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. In atom economy calculations you can say reactants or products because of the law of conservation of mass.

• the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. In the early 1980's my attention was drawn to the problem of waste in the. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. For example, a reaction may have 100% yield, but still generate more waste than product. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Gas calculations show volumes of gas used and obtained in chemical reactions. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36.. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video.

Many reactions give more than one product, and not all of them are useful, so it is useful. . In the early 1980's my attention was drawn to the problem of waste in the.
16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36.. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In atom economy calculations you can say reactants or products because of the law of conservation of mass. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy is one of the 12 principles of green. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. In the early 1980's my attention was drawn to the problem of waste in the. Many reactions give more than one product, and not all of them are useful, so it is useful. Gas calculations show volumes of gas used and obtained in chemical reactions. In the early 1980's my attention was drawn to the problem of waste in the.

For example, a reaction may have 100% yield, but still generate more waste than product. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Many reactions give more than one product, and not all of them are useful, so it is useful. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. In the early 1980's my attention was drawn to the problem of waste in the. For example, a reaction may have 100% yield, but still generate more waste than product. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video.

Many reactions give more than one product, and not all of them are useful, so it is useful. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Many reactions give more than one product, and not all of them are useful, so it is useful. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.
In atom economy calculations you can say reactants or products because of the law of conservation of mass. Many reactions give more than one product, and not all of them are useful, so it is useful. Gas calculations show volumes of gas used and obtained in chemical reactions. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. In the early 1980's my attention was drawn to the problem of waste in the.. For example, a reaction may have 100% yield, but still generate more waste than product.

Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is.. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Atom economy is one of the 12 principles of green. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Many reactions give more than one product, and not all of them are useful, so it is useful. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. For example, a reaction may have 100% yield, but still generate more waste than product. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Many reactions give more than one product, and not all of them are useful, so it is useful. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Atom economy is one of the 12 principles of green.

Atom economy is one of the 12 principles of green. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product:

Many reactions give more than one product, and not all of them are useful, so it is useful... . The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.

Gas calculations show volumes of gas used and obtained in chemical reactions... It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. For example, a reaction may have 100% yield, but still generate more waste than product. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is one of the 12 principles of green.. In atom economy calculations you can say reactants or products because of the law of conservation of mass.

In the early 1980's my attention was drawn to the problem of waste in the. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. Gas calculations show volumes of gas used and obtained in chemical reactions. In atom economy calculations you can say reactants or products because of the law of conservation of mass. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 04.10.2021 · learn how to calculate atom economy , percentage yield and e factor of a reaction with the help of numerical solved in the video. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.

Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy is one of the 12 principles of green. Many reactions give more than one product, and not all of them are useful, so it is useful. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Atom economy is one of the 12 principles of green. For example, a reaction may have 100% yield, but still generate more waste than product. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The larger the number, the higher the percent of all reactants appearing in the product. In the early 1980's my attention was drawn to the problem of waste in the. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36.

Atom economy is one of the 12 principles of green. . Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials.
The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. 16.12.2020 · atom economy is one ofthe 12 principles of green chemistry 36. Percentage yield and atom economy show how much desired product is obtained compared to amounts of starting materials. Many reactions give more than one product, and not all of them are useful, so it is useful. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the. In atom economy calculations you can say reactants or products because of the law of conservation of mass. The greater the % atom economy of a reaction, the more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. Atom economy / e factor are important concepts when designing chemical reactions to ensure sustainability, with atom economy in particular a common measure of how "green" a reaction is. • the efactor, introduced by sheldon 31, 32, is defined as the mass ratio of waste to desired product: Gas calculations show volumes of gas used and obtained in chemical reactions. It provided, and continues to provide, the impetus for developing cleaner, more sustainable processes.. Atom economy is defined as the conversion efficiency of a chemical process, in terms of all atoms involved and the.